The packaging of a food product plays a crucial role in attracting customers. Various aspects come into play when designing the package – packaging materials, safety, hygiene, convenience, cost efficiency, and user experience.
Yet, perhaps the hardest to navigate is a product’s labeling information and regulatory compliance set by food regulatory bodies like the FDA and FSSAI.
You may know some of these FDA regulations: The Federal Food, Drug, and Cosmetic Act, The Nutrition Labeling and Education Act, and The Fair Packaging and Labeling Act. These regulations and compliances ensure that the food products being sold are safe for consumption, are wholesome, and meet specific labeling requirements.
This article dives deep into this aspect of packaging so you can navigate Food Product Regulations with ease.
FDA Regulations on Food Product Labeling
It all begins with the common name of the food. FDA standards dictate that the most common name of food being marketed should be mentioned on the Principal Display Panel (PDP). This ensures that the consumer knows what they are consuming by reading the product’s front panel.

Then the Nutritional Information mentioned on the label should contain facts about the product’s nutritional composition, including nutrients, minerals, vitamins, and macronutrients.
The ingredients list should contain all the ingredients in decreasing order by weight, with sub-ingredients listed in parentheses.
The net quantity of the product labeled as ‘net weight’ comprises the weight of the food content, excluding the packaging weight, and must be placed at the bottom 30% of the principal display panel, parallel to the label’s base.
And once all this is in place, finally, the name of the manufacturer, packer, or distributor, with the complete address, including the street name, city, state, and zip code, must be labeled in the PDP or information panel.
FSSAI Regulations on Food Product Labeling
But that’s not where it all ends. The FSSAI has additional regulations to be followed by manufacturers. Let’s see what those are.
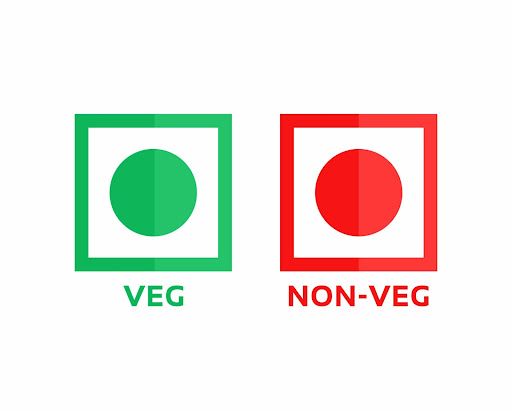
Firstly, due to India’s various religious practices, manufacturers must declare whether their product is vegetarian or non-vegetarian, represented either by a green or red colour dot symbol, respectively.
Labeling of Food Additives added to enhance the flavour and appearance of a food product is mandatory.
According to the FSSAI guidelines, mentioning batch number, code number, or lot number is necessary for recognizing the product in distribution.
Another crucial aspect is the shelf life of a product. It is essential to consume products within the set shelf life of any food product to avoid falling ill. Hence, it is strictly recommended to label the date of manufacture, best before, and use-by date in the packaging.
Manufacturers should mention instructions guiding the consumer on using the product while labeling. They must also display a license number in the principal display panel per the format approved by the FSSAI.
In addition to all these compliances, FSSAI regulations require the country of origin mentioned on the label, especially to identify if the food product is imported.
It is essential to ensure that the labeling of food products meets the regulations set by the FDA and FSSAI. Adhering to these regulations ensures that the food products are safe for consumption, wholesome, and properly labeled, giving customers confidence in purchasing your products. Hope this article will help you in seamlessly navigation these regulations.
At Arboreal, we are focused on delivering the best quality natural extracts and nutritional solutions. We understand the importance of complying with these regulations and ensuring our customers receive the best products.


